![]()
Writing a research proposal for post graduate degree dissertation by a post graduate medical student
Article Type: Short Communication
Year: 2024; Volume: 4; Issue: 4; Page No: 29 – 34
![]() https://doi.org/10.55349/ijmsnr.2024442934
https://doi.org/10.55349/ijmsnr.2024442934
Affiliations: Assistant Professor in Statistics (Biostatistics), Department of Community Medicine, Sri Venkateshwaraa Medical College Hospital & Research Centre, Ariyur, Pondicherry – 605 102. Email ID: senthilvel99@gmail.com
| How to cite this article: Vasudevan S. Writing a research proposal for post graduate degree dissertation by a post graduate medical student. Int J Med Sci and Nurs Res 2024;4(4):29–34 . DOI: https://doi.org/10.55349/ijmsnr.2024442934 |
Corresponding Author:
Dr. Senthilvel Vasudevan, Ph.D,
Assistant Professor in Statistics (Biostatistics),
Department of Community Medicine,
Sri Venkateshwaraa Medical College Hospital & Research Centre,
Ariyur, Pondicherry – 605 102. India.
Email ID: senthilvel99@gmail.com
ORCID: https://orcid.org/0000-0001-7175-3534
Article Summary: Submitted: 20-October -2024; Revised: 25-November-2024; Accepted: 15-December-2024; Published: 30-December-2024
Abstract
Background: Research proposal writing is needed a keen interested and knowledge in their specialty. It is very important and essential one for any type of research in science and in any field. Their dissertation work only helps/gives a good solution to know a new–line of treatment. I have discussed about the various steps in research methodology, preparation of a research proposal, how to conduct/proceed a dissertation and how to write a dissertation/study.
Materials and Methods: I have discussed I this paper related various steps in research methodology like research topic, research design, target population, sample size and how to calculate it, how to frame inclusion and exclusion criteria, how to enter the data in excel sheet, statistical analysis, what is one sided and two sided tests, how to frame a null and alternative hypothesis, how to conduct/proceed a dissertation and how to write a dissertation/study, What is type-I ad Type-II errors, level of significance, and the process of Scientific Research Committee and Institutional Ethical Committee Approval for a post graduate medical student’s research proposal.
Results: All the explanation is to be given in a elaborated ways. The post graduate medical students have to understand easily.
Conclusion: From this article, I have concluded that post graduate medical students have to understand and try to follow all the steps of research methodology at the time of their medical degree dissertation proposal preparation.. Adequate minimum calculated sample size should be determined to get a significant results in their studies. Getting SRC ad IEC approval for their dissertation work is a mandatory process in the medical curriculum. Finding the sample size for their PG degree dissertation at the time of preparation of their research proposal.
Keywords: post graduate medical student, research methodology, level of significant, type-I and type-II errors, proposal, dissertation
Full Text
Introduction
Research proposal writing [1-2] is an important art of a Post-Graduate Medical Students/researcher/scientist. It is very important and essential one for any type of research in medical science, and in any field. All post-graduate medical students are preparing their research proposal for their dissertation project, short study and other types of studies in their study period. This type of exercise is educating the students in the basics of research methods, methodology, how to writing/express the scientific writing and to encourage the medical students/students to increase their thinking in their field and also increase the students’ critical thinking in their area of science. For Post-Graduate Medical Students (PGMS), their dissertation is very much important in their study. This is mandatory as per the National Medical Commission (NMC). [3] Because, once they have finished and submitted their research dissertation then only they are eligible to attend their final degree examination. Any PG medical students have to do their dissertation research works under a supervision/guidance of a guide those who are working as Assistant/Associate Professor or Professor/Additional Professor grade and determine the sample size with a help of well-trained statistician/ biostatistician/ tutor/ Assistant/ Associate Professor/ Professor of Statistics. Because the guide is the main and important role in doing their PG dissertation research work.
Any PG Medical Student has to do any kind/type of research; they have to conduct a statistician at the time of preparing of their research proposal. This is very important procedure in doing a research/study/project in their specialty. Because, all the aspects like title, primary and secondary objectives, inclusion and exclusion criteria, methodology, statistical analysis, other important things to be discussed and finalize at the time of preparing their research proposal with the help of Statistician/Biostatistician. Once the PG Medical Student has to do a study in a proper manner then they will have to become a good researcher and their dissertation work will help to them to know about the various steps of research methodology related to their study. Also it will help in acquiring may other skills like critical thinking, reasoning, and analytical skills in their PG studies. In this article, After finishing their PG medical studies, many of them who never do any kind of research in their profession due to work load or lack of time to spend in the research/study. Their dissertation work only helps/gives a good solution to know a new–line of treatment in their particular/any specialty in the medical field available in the literature/database for the benefit of a particular diseased patient. I have discussed about the various steps in research methodology, preparation of a research proposal, how to conduct/proceed a dissertation and how to write a dissertation/study.
Preparation of protocol for Post-Graduate Graduation Study:
Document of a research results conducted by a Post-Graduate Medical Student under a proper guidance of a guide, present it in a national /international conference and then publishing that particular research work. Writing a proper PG Medical dissertation is most important thing is to present the research findings in a proper and acceptable manner/format. The submitted dissertation is also reviewed by three examiners to determine a part of the criteria for the candidate to pass the Masters’ Degree Medical Examination as per medical education curriculum. [3]
The important role in a preparation of dissertation/thesis work is that of the guide who has to mentor his proteges through the process by educating them on the various steps of research methodology. They have to prepare their research proposal in following steps: (a). Identifying a unique and prominent research question for a study; (b). To formulate the type of study and the design of study for their research/study/project; (c). Identifying the study population; (d). Calculating the adequate sample size based on some appropriate formula; (e). Collecting and compiling the data related to the particular study correctly; (f). How to analyze the entered data in the excel by existing statistical software; (g). Concluding the study/research by scrutinizing the analyzed research outcome, (h). To make the research findings to the reader/public by publishing in an acceptable manner in a peer-reviewed national/international journals. Sometimes, the co-guide will be fixing and perform as a co-investigator from the same or another department related to the study topic. The co-guide/Co-investigator will work as an equivalent role in guiding the student in their dissertation work. [4]
Research is an independent creative and fantastic work by a research to increase the knowledge. The dissertation work has to be prepared in the following topics/headings: Introduction, Aim of the Study, Objectives of the study, Materials and Methods: Describing important terms, Description of devices if any or pharmacology of drugs, Review of Literature, Observations and Results, Discussion, Conclusions, Limitations of the study, new abbreviations (if any), Bibliography, Proforma/Questionnaire, Master chart. Some necessary certificates from the guide and the institute are a requirement in certain universities. The students often add an acknowledgement page before the details of their dissertation proper. It is their expressions of gratitude to all of those who they feel have been directly or indirectly helpful in conduct of the study, data analysis, and finally construction of the dissertation. [4-5]
Choosing a suitable article as key article:
First PGMS has to decide their dissertation topic/research title with the help of their respective guide. After that only, they have to search and find out the key/parent article. The parent article is a similar article at least 50% – 60% with their respective research title. This is very important one and mandatory procedure. Sometimes, PGMS hasn’t found their key/parent article suitable/appropriately. At that time, they have to find some of values related mean and standard deviation then based on these two values they would have to find their adequate sample size for their particular study / research / project.
If the student hasn’t found their similar article (parent article) from the previous existing literatures via PubMed, MedLine and other databases like Google Scholar, SCOPUS, then they will consider their study as pilot study. [5]
Review of Literature:
This review of literature part is very much important in the PG medical dissertation work. All PG students should have keep the following important things at the time of preparing their research proposal. (a). Collect all the relevant and existing articles related to their research question/problem. (b). Find the authors name, their affiliations, which year they did that study? What methodologies they used in their study? What theories they used in their study? What are all their findings in that particular study? What are all the authors would have been used the similar types of methods ad arrived similar types of results in the existing literature. (c). Collect all the approaches, themes, methods, and controversies expressed by various authors in the exiting literature. What are all the major areas of disagreements and why? What are all the different findings and debate? (d). Which approaches are coincide with their preset study, what are all the findings ad methodologies seem most reliable, valid and appropriate one and why? Very much careful to the verbs you use to describe what a author says or does? (e). The review literature collection is similar to your area of research ad investigation. ie., How is your research work to e proceed? Whether the previous methods are suitable for your study (or) it would be modified or improved in the methodology wise. [3-5]
Formation of primary and secondary objectives:
Primary Objective:
Forming their primary objective to discuss with their guide and finalize it. This is a unique one which is exactly similar to their study main finding. This is to be decided with keen respective subject knowledge related to the medical specialties. Because the study sample is to be determined mainly with help of this only.
Suppose, PG dissertation is mainly concentrating on prevalence means then the word prevalence is in their research title as well as in their primary objective also. This is mandatory because of that based on primary objective only, they should have to calculate the sample size for their present research.
Secondary Objectives:
This is also a very important part in objective making in any medical/science studies. This part contains one/two/three in number based on their research/study/project. The secondary important outcome/finding is to be mentioned in this part.
Sometimes, the medical students have to mention the primary objectives in the secondary objective part. That is to be finding and rectified by their respective guide or in the Scientific Research Committee at the time of the primary research proposal presentation. [1-2, 6]
Sample size formula for various kinds of studies: [6-9]
Sample size calculation and fixation is an important and essential step in the conduct of medical research/Clinical study. It is a critical step and difficult to understand. Because different methods of sampling procedure to be used for different types of study designs. Using the following link to the sample size calculation/estimation in clinical research: https://riskcalc.org/samplesize/
After finding the sample size for their research title then, they have to reporting the sample size determination in a correct way. This is very important. The existing various types and sub types of study designs in medical/clinical research as shown in Figure-1.
Figure–1 Flowchart of various types and sub types of study designs in medical/clinical research
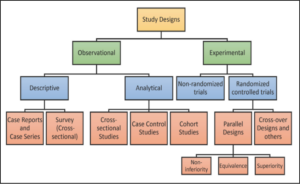
Source: Cleveland Clinic – Department of Quantitative Health Sciences
Null Hypothesis and Alternative Hypothesis:
These two things are coming under hypothesis testing. Two mutually exclusive statements about a study population are null and alternative hypotheses. In that, null hypothesis is denoted by H0. Which is always to be rejected. It means the opposite of what results to be proved/needed by the researcher/investigator. Another one is alternative hypothesis and it is denoted by H1 (or) Halt. It means a statement that proved a potential results expected by the researcher.
The general statements of null and alternative hypotheses are as follows:
H0: There is no significant difference between the two groups/ populations/ samples/ drugs/ treatments/ methods
H1: There is some significant difference between the two groups/ populations/ samples/ drugs/ treatments/ methods
Sometimes, H0 is true; but it would be rejected
In a study a researcher, H0 is true and it would be rejected. What it is meaning? H0 general statement is: There is no significant difference between the two groups. Once it is to be rejected means then the researcher has to accept the H1 or Halt automatically. It means, some statistical difference is there in between the groups/ / populations/ samples/ drugs/ treatments/ methods. Otherwise vice versa.
Hypothesis testing is used the sample data to determine whether the hypothesis to be reject the H0 or not. Suppose not being to reject the H0 it doesn’t mean that it is true. We don’t have adequate evidence to reject it. That’s why; adequate sample size is needed to any type of studies in medical/any type of science studies.
Mean, Standard Deviation and Standard Error of Mean:
Mean, standard deviation (SD), and standard error of mean (SEM) values are very important measures for each and every medical related studies. Mean age of the study participant/patients/students from their collected data is very much important and essential one. Nowadays, in some of the medical research/studies the researchers haven’t mentioned about the mean, SD, and SEM in their studies. These three measures have explained about the characteristics of the study sample and as well as about the results of statistical analysis. Standard deviation value is very small one is the correct measure. If any research had to receive their SD value is very high value then, the deviation between their collected data is also very high. This is the meaning. So, data collection method is very important step in a conducting a research/study.
If we square the SD (σ) value then we will get the variance value. SD is also called as “Root Mean Square Deviation”. This SD value would be get from their existing literature (or) from their pilot study. If study primary outcome of their medical research is a binary one, the SD value isn’t required for the calculation process of sample size. SD is the dispersion of data in normal distribution.
In may medical researches/literature mentioned/represent mean along with SD or SEM in the report of statistical analysis results. [7]
For example: Suppose, Sample: x1, x2, x3, x4, x5, … … .. , xn
Sum of all given observations
Mean = ————————————-
Number of given observations
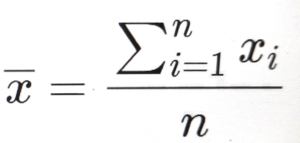
SD = σ = √(∑( x − x ¯ ) 2 /n – 1) If n < 30
SD = σ = √(∑( x − x ¯ ) 2 /n) If n > 30
Standard Error of Mean (SEM) = σx̄ =
s
—–
√n
Statistical Power (1 – β):
Statistical power is very much important in any type of studies. Always the statistical power is considering as 80%. Statistical power is a tool to measure to conduct an experimental study and hypothesis testing setup to find out a particular effect if it is truly present or not. Otherwise, statistical power is the measure that a study will correctly detect an effect (or) reject a false null hypothesis. It is a very much difficult process that during the time of planning a study and in the stages of designing. Beta value (β) is the probability of making a Type II error and it is an inverse relationship with the statistical power (1 – β). If the risk of committing a Type II Error (β) value is 20%, then the statistical power would be (100% – 20%) = 80%. Always statistical power to be fixed as 80% in all medical and science studies. [10]
Statistical power is affected by the following things:
- . Sample Size
- . Minimum Effect of Interest
- . Significance Level (α)
- . Desired power level (Type II Error rate)
One Sided and Two Sided Tests
Figure-2 Showing the diagram of One Sided and Two Sided Tests
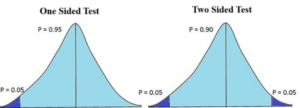
One Sided Test: The one sided test denotes the direction of alternative hypothesis. ie., It is to determine whether the parameter of the population whether greater than or less than the hypothesized value.
Example: Blood pressure value of a patients whether increases or decreases over time by the newline of treatment.
One sided tests are following asymmetric distributions with a single tail. ie, right skewed or left skewed.
Two Sided Test:
In the two sided test, no direction be specified by the alternative hypothesis. Example: The average height is not 160 cms as shown in Figure – 2.
Type-I and Type-II Errors:
Type-I Error
Type I Error is happening when the null hypothesis true but, it should be rejected. It is called as False Positive. Type I Error rate is also called “Level of Significant”. ie., the probability of rejecting the null hypothesis (H0) when it is true. It is also called as “Alpha Level” and it is denoted by the symbol “α”. Level of significant (α) level always to be fixed as 5% (or) 0.05 for all the studies in medical field. It implied that it is acceptable to have a level of significant 5% and if greater than 5% it would be rejecting the null hypothesis as shown in Table – 1.
Type-II Error
Type II Error is happening when the null hypothesis false but, it should be accepted. It is called as “False Negative”. Its rate is the probability that the null hypothesis is to be rejected when it is false. It is denoted by the symbol (1–β). Normally, the statistical power is set as 80% and sometimes 90% at the time of calculating sample size for a medical/science related studies as shown in Table – 1.
Table – 1 Showing types of errors, null hypothesis ad its decision [11]
| Types of Errors | Null Hypothesis (H0) | ||
| True | False | ||
| Decision about Null Hypothesis (H0) | Accept | Correct Inference (true negative) | Type II Error
(False Negative) (probability = β) |
| Reject | Type I Error
(False Positive) (probability = α) |
Correct Inference
(True Positive) (probability = 1 – β) |
|
Level of Significant:
Significant level always to be fixed as 5% for all the studies in medical field research and two tail test would be considered. But, in the clinical trail the minimal detectable difference ie., 1% or 0.01 implemented to the smallest difference between treatments that is considered as significant clinically. Various levels of significance and its specifications as shown in Table – 2.
Table – 2 Showing various levels of significance and its specifications [12]
| Various levels of significance | Specifications |
| p > 0.05 | Not Significant |
| p ≤ 0.05 | Significant |
| p = 0.05 | Merely Significant |
| p ≤ 0.01 | Highly Significant |
| p ≤ 0.001 | Very Highly Significant |
Scientific Research Committee and Institutional Ethical Committee Approval:
After preparing the research proposal by the Post Graduate Medical Students those who would have been applied for the approval and presented in the Scientific Research Committee (SRC). The student has to make the necessary changes/corrections by SRC. Then the PGMS should has to apply for the Institutional Ethical Committee (IEC). After that, they have received the essential corrections by IEC, according to that they have changed all the corrections. After that only the PG medical students have to start their data collection. This will be mentioned as “Ethical Approval and Statement” at the last 2 or 3 three in the methodology part.
Conclusion
From this article, I have concluded that post graduate medical students have to understand and try to gain/learn deep knowledge and familiar in all the steps of research methodology. Adequate minimum calculated sample size should be determined or calculated based on their primary objective of their respective medical studies. If not then, they wouldn’t get a significant results in their studies. Getting SRC ad IEC approval for their dissertation work is a mandatory process in the medical curriculum. Usually, the statistical power is to be set as 80% and sometimes 90% at the time of calculating sample size in any type of medical/any science related studies. All post graduate medical students have to read and follow of all the steps of research methodology at the time of their medical degree dissertation proposal preparation. Finding the sample size for their PG degree dissertation after the preparation of their research proposal. Fix an Assistant Professor in Statistics (or) Biostatistics as a co-guide for the medical research is best solution to avoid maximum statistical errors in their post graduate medical degree dissertation work. This initial exercise of writing a research proposal is very much helpful to the PG Medical Students to do their Ph.D in their respective medical specialty.
Source of funding: None
Conflict of Interest: Nothing to declared by the author
Authors’ Contributions: SV – Author contributed to the conceptualization, writing of the article and in preparation and checking of the article.
Here, SV – Senthilvel Vasudevan
References
- McGranaghanM. Guidelines on Writing a Research Proposal. Availale from: https://www2.hawaii.edu/~matt/proposal.html [Last Accessed on: 18th June 2024]
- University of Sidney: How to write a research proposal: A guide to preparing a strong research proposal. Available from:https://www.sydney.edu.au/study/applying/how-to-apply/postgraduate-research/how-to-write-a-research-proposal-for-a-strong-phd-application.html [Last Accessed on: 20th June 2024]
- NationalMedical Commission: Clarification on the mandatory requirement of dissertation/thesis submission. Available from: https://www.nmc.org.in/MCIRest/open/getDocument?path=/Documents/Public/Portal/LatestNews/33%20Cover%20letterd_merged.pdf [Last Accessed on: 22th June 2024]
- Vasudevan S. Usage of Statistics tools in the area of Pharmacological research. Int J Med Sci and Nurs Res 2023;3(4):8- DOI: https://doi.org/10.55349/ijmsnr.202334810
- Jawaharlal Institute of Postgraduate Medical Education & Research: Postgraduate dissertationGuidelines 2020 (Approved in the 15th SAC meeting held on July 2020. Available from: https://jipmer.edu.in/sites/default/files/PG%20Dissertation%20Guidelines%202020%281%29.pdf [Last Accessed on: 25th July 2024]
- Vasudevan S. Sample Size Calculation in Various Medical Research. Int J Med Sci and Nurs Res 2024;4(3):22–29. DOI:https://doi.org/10.55349/ijmsnr.2024432229
- Vasudevan S, Aishwariya R. Menstrual health ad hygiene practices of adolescent girls attending school in rural parts of South India and its effect on school attendance in the year 2020: A Descriptive Cross-Sectional Study. Int J Med Sci and Nurs Res 2024;4(3):6-14 DOI: https://doi.org/55349/ijmsnr.202443614
- Mustafa SH, Mohammed EA, Salih AM, Kanagarajan P, Albushra MMO, Makkawi ST, Mustafa AH. A Qualitative Exploration of Acceptance of a Conversational Chatbot as a Tool for Mental Health Support among University Students in Sudan. Int J Med Sci and Nurs Res 2024;4(1):16-23. DOI: https://doi.org/55349/ijmsnr.2024411623
- Hasan RT, Salman AD. Assessment of Women’s Knowledge and Attitude towards Modes of Delivery in Al-Elwia Maternity Teaching Hospital in Iraq: A Descriptive Study. Int J Med Sci and Nurs Res 2024;4(1):5–10. DOI:https://doi.org/10.55349/ijmsnr.202441510
- Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb) 2021;31(1):010502. DOI: https://doi.org/11613/BM.2021.010502 Epub 2020 Dec 15. PMID: 33380887; PMCID: PMC7745163
- Banerjee A, Chitnis UB, Jadhav SL, Bhawalkar JS, Chaudhury S. Hypothesis testing, type I and type II errors. Ind Psychiatry J 2009;18(2):127-1 DOI:https://doi.org/10.4103/0972-6748.62274 PMID: 21180491; PMCID: PMC2996198.
- García-Berthou E, Alcaraz C. Incongruence between test statistics and P values in medical papers. BMC Med Res Methodol 2004;4:13. DOI: https://doi.org/1186/1471-2288-4-13 PMID: 15169550; PMCID: PMC443510.
![]() This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
