![]()
Efficiency of expressed breast milk versus Dextrose administration on pain during blood sampling in neonates – A Comparative Study
Year: 2025; Volume: 5; Issue: 1; Page No: 14 – 19
Article Type: Original Article
Authors: Brinda P1*![]() , Reetha KF2
, Reetha KF2![]() , Keka Chatterjee 3
, Keka Chatterjee 3![]() , Saritha Sudharma4
, Saritha Sudharma4![]()
![]() https://doi.org/10.55349/ijmsnr.2025511419
https://doi.org/10.55349/ijmsnr.2025511419
Affiliations:
1Lecturer, College of Nursing, INHS Asvini, Colaba, Mumbai, Maharashtra, India.
2Ward Sister, INHS Asvini, Colaba, Mumbai, Maharashtra, India.
3 Vice Principal, College of Nursing, INHS Asvini, Colaba, Mumbai, Maharashtra, India.
4 Principal, College of Nursing, INHS Asvini, Colaba, Mumbai, Maharashtra, India.
| How to cite this article: Brinda P, Reetha KF, Chatterjee K, Sudharma S. Efficiency of expressed breast milk versus Dextrose administration on pain during blood sampling in neonates – A Comparative Study. Int J Med Sci and Nurs Res 2025;5(1):14–19. DOI: 10.55349/ijmsnr.2025511419 |
Corresponding Author:
Ms. Brinda P,
Lecturer, College of Nursing,
HIS ASVII,
Mumbai.
Maharashtra, India.
Email ID: brindzpure@rediffmail.com
Article Summary: Submitted: 10-January-2025 Revised: 15-February-2025 Accepted: 20-March-2025 Published: 31-March-2025
Abstract
Background: Pain is a state frequently experienced in neonatal period during illness and invasive interventions. The stress that emerges from recurrent painful procedures might lead to deterioration in clinical condition as well as potential long-term negative consequences. Hence assessment of pain and provision of pain relief should be among the primary interventions. The main objectives were to assess the pain scores in experimental group-I and experimental group-II, to identify the efficacy of expressed breast milk vs 25% dextrose in pain relief during blood sampling in neonates, and to identify the association of sociodemographic variables with pain scores in both groups
Materials and Methods: A pre-experimental design using convenient techno following pre-designed inclusion & exclusion criteria among 60 neonates was done. Informed consent was taken from parents. Ethical clearance and permission from administration was obtained. A standardized tool (NIPS) was used to assess the pain during blood sampling. Pilot study was conducted and samples of the pilot study were excluded from the main study. Expressed breast milk group was assigned as the experimental group-I & 25% dextrose group was assigned as experimental group-II.
Results: The mean pain score of group-I was 3.73 ±1.55, in contrast to the group-II 2.67±0.96 indicating that pain experienced by neonates in group-II was lower than that in group-I. Independent t-test was done. The findings were statistically significant with t = -2.52, p=0.014.
Conclusion: This study reveals that 25% dextrose administered orally is effective in reducing pain during blood sampling in neonates as compared to expressed breast milk.
Keywords: expressed breast milk, 25% Dextrose, efficacy, blood Sampling, tertiary care hospital
Full Text
Introduction
Pain is a state frequently experienced in neonatal period during illness and invasive interventions. [1] The stress that emerges from recurrent painful procedures might lead to deterioration in clinical condition as well as potential long-term negative consequences. In the realm of neonatal care, effective pain management strategies are paramount to ensure the well-being and comfort of infants undergoing various medical procedures. Despite research on pharmacological interventions, non-pharmacological interventions, like breastfeeding or expressed breast milk are employed to provide comfort and pain relief through the release of endorphins and promotion of a soothing environment. Expressed breast milk and dextrose remains an area of ongoing investigation and has gained attention for its potential analgesic properties. [3 – 8]
Expressed breastmilk, a natural substance rich in nutrients and bioactive compounds, is not only vital for infant nutrition but also posited to possess pain-relieving qualities. Concurrently, commercially available dextrose, a simple carbohydrate, has been explored for its ability to mitigate pain responses in neonates undergoing painful procedures. By synthesizing current literature and empirical evidence, this study seeks to contribute to insights into evidence-based practices and enhance the quality of care provided to neonates undergoing painful procedures. [9 – 14]
The main objectives were to assess the pain scores in experimental group-I and experimental group-II, to identify the efficacy of expressed breast milk vs 25% dextrose in pain relief during blood sampling in neonates, and to identify the association of sociodemographic variables with pain scores in both groups.
Material and Methods
Study Design, Setting and Duration of the Study: A Pre-experimental study was conducted in a tertiary care hospital in Maharashtra between March 2024 and August 2024.
Study Population: Neonates who undergo painful procedures in various tertiary care hospitals
Study Participants: Neonates admitted in postnatal wards and neonatal intensive care units born between 34 and 42 weeks of gestation.
Study Variables: Dependent variable: pain perceived by neonates during blood sampling procedure. Independent variable: Administration of expressed breast milk vs 25% dextrose before blood sampling.
Sample Size Calculation: Sample size was calculated as 60. A power of 90% was set, at two-sided significance level (α) of 5%, the required sample size per group was calculated based on the formula for comparing two independent means:
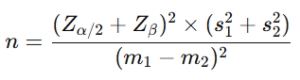
where Zα/2 = 1.96 for a 5% significance level, Zβ = 1.28 for 90% power, and s1 and s2 are the standard deviations (Charan & Biswas). [30] The calculated sample size was 27 neonates per group, which was rounded up to account for potential dropouts. Thus, a total of 60 neonates were enrolled, with 30 receiving to 30 per group expressed breast milk (EBM) and 30 receiving 25% dextrose, following an equal allocation ratio of 1:1.
Inclusion Criteria included neonates aged ≤28 days, gestational age 34–42 weeks, and scheduled venue puncture.
Exclusion Criteria included neonates with sepsis, sedation, congenital anomalies, or crying prior to the procedure. Protocol was drawn and validated.
Study Tool: A standardized tool Neonatal Infant Pain scale was used record the pain scores.
Ethical Clearance: Permission obtained from Institutional Ethical Committee (IEC No: 85/2024)
Method of Data Collection: Convenience sampling identified 60 neonates, divided into two groups: Group-I was administered 2 mL of expressed breast milk orally and Group-II was administered
2 mL of 25% dextrose orally. Neonates who fit the inclusion criteria were included in the study after obtaining informed written consent from the parents. Gestational age was confirmed with modified Ballard scoring. All neonates subjected to the study were ensured that they were adequately fed 30 min before the intervention.
Protocol of the study: Before 30 s of venipuncture, one group of neonates was orally administered 2 mL of 25%D by 2 mL sterile syringe and the other group was orally administered 2 mL of EBM by 2 ml sterile syringe of the respective mother by the principal Investigator. With the baby lying on the radiant warmer, 30s after administration of 25%D or EBM, a venipuncture was done and response to procedural pain was assessed by NIPS Scoring.
Data management & Statistical Analysis: Data was computed by using Microsoft Excel 2010 and analysed using IBM SPSS 27.0 version. Descriptive statistics (Frequency & percentage) summarized demographic data; Independent t-test/Mann Whitney U test was used for inferential statistics. Level of significance was set at p-value<0.05.
Results
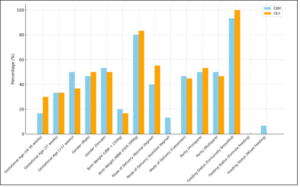
Distribution of samples in both the interventional groups with the selected sociodemographic variables. The study population included neonates with a mean gestational age of 37 weeks, with both genders represented, Birth weights ranged from approximately 1.8 kg to 3.5 kg, maximum samples (80-82%) were with birth weight of 2.5 to 3.0 kg. Neonates were delivered through various modes, including normal vaginal delivery, caesarean section, and assisted vaginal delivery, reflecting a balanced representation of delivery methods. Regarding parity, both primiparous (first-time mothers) and multiparous mothers contributed to the study sample. The feeding status of neonates shows that maximum neonates were exclusively breastfed (>90%) as shown in Figure–1.
Figure–1 Distribution of sociodemographic variables among groups (N=60)
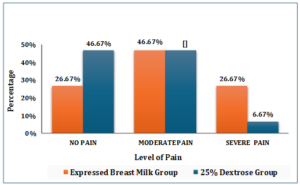
The group of neonates who received 2 ml of expressed breast milk during blood sampling, the majority, 14 (46.67%), experienced moderate pain. This was followed by 8 neonates (26.67%) who experienced no pain, and another 8 neonates (26.67%) who experienced severe pain. In contrast, among neonates administered 2 ml of 25% dextrose during blood sampling, the majority, 14 (46.67%), experienced no pain and equal number, 14 (46.67%), reported moderate pain, while only 2 (6.67%) neonates experienced severe pain as shown in Figure–2.
Figure–2 Distribution of pain scores in Experimental group-I & Experimental group-II (N=60)
The association between socio-demographic variables and pain & was analyzed using the Mann-Whitney U test for two-group comparisons. There was no statistically significant association with gestational age and pain (p>0.05), indicating that pain responses were similar across different gestational age groups. Also, there was no significant difference in pain levels between male and female neonates (p>0.05). Similarly, birth weight showed no significant association with pain (p>0.05). The mode of delivery (normal vaginal, assisted vaginal, and cesarean section) did not significantly influence pain (p>0.05), nor did parity (primiparous vs. multiparous mothers) (p > 0.05). Feeding status, comparing exclusively breastfed neonates with mixed feeding, also showed no significant association with pain (p>0.05). Overall, none of the analyzed socio-demographic variables were found to have a significant impact on pain as shown in Table–1.
Table–1 Association of socio-demographic variables and pain relief during blood sampling (N=60)
|
Socio-Demographic Characteristics |
Expressed Breast Milk (N=30) |
25% Dextrose (N=30) |
||
|
Mean ± SD |
K/U value | Mean ± SD |
K/U value |
|
| Gestational Age (in Weeks) | ||||
| 34 to 36 |
4 ± 1.52 |
0.082K |
3 ± 1.05 |
0.118 K |
|
37 |
3 ± 1.20 |
2 ± 0.63 |
||
| Above 37 |
4 ± 1.62 |
3 ± 1.01 |
||
| Gender | ||||
| Male |
4 ± 1.47 |
0.294U |
3 ± 0.80 |
0.920 U |
|
Female |
4 ± 1.63 |
3 ± 1.12 |
||
| Birth Weight (in Grams.) | ||||
| LBW: Less than 2,500 |
4 ± 1.37 |
0.230 U |
3 ± 1.22 |
0.590 U |
| NBW: 2,500–3,999 |
4 ± 1.59 |
3 ± 0.91 |
||
| Mode of Delivery | ||||
| Normal Vaginal Delivery |
3 ± 1.53 |
0.167 K |
3 ± 0.98 |
0.680 U |
| Assisted Vaginal Delivery |
5 ± 1.73 |
̶ |
||
| Caesarean Section |
4 ± 1.47 |
3 ± 0.97 |
||
| Parity | ||||
| Primipara |
4 ± 1.79 |
0.775 U |
3 ± 0.81 |
0.790 U |
| Multipara |
4 ± 1.30 |
3 ± 1.14 |
||
| Feeding Status | ||||
| Exclusively Breastfeed |
4 ± 1.54 |
0.556 U |
3 ± 0.96 |
̶ |
| Mixed Feeding |
5 ± 2.12 |
̶ |
||
The Independent t-test comparing the mean NIPS scores between the dextrose and EBM groups. It reveals a test statistic value of t = -2.52 at degree of freedom 57.81. The p-value was calculated as 0.014 which is less than the level of significance of 0.05, indicating that there is statistically significant difference in pain scores between the two groups. This suggests that Dextrose was more effective than Expressed Breast Milk in reducing pain levels in neonates as shown in Table–2.
Table–2 Comparison of mean pain scores between groups (N=60)
|
Sl. No. |
Groups | Sample Size (n) | Mean | SD | t-statistic | df | p-value |
| 1 | EBM | 30 | 3.40 | 1.26 | -2.52 | 57.81 |
0.014 Sig. |
|
2 |
25% D | 30 | 2.67 |
0.96 |
SD – Standard Deviation; df – Degrees of freedom; p<0.05 and Sig. – Significance
Discussion
The 25% dextrose is sweet, sterile, readily available at low cost in NICU and postnatal ward. EBM was selected as it is a natural disaccharide readily available and has less chance of contamination and infection. The findings of this study indicate that 25% dextrose provides significantly better pain relief compared to expressed breast milk during neonatal blood sampling. This is consistent with previous research by Rawal et al [8] and Sahoo et al [9], which highlighted the efficacy of sweet solutions in activating endogenous opioid pathways. In contrast, Upadhyay et al [12] and Mei-Chen Ou-Yang et al [20] found that expressed breast milk reduces pain associated with heel lancing in preterm neonates.
The use of 25% dextrose as a pain relief option is supported by studies that have shown its effectiveness in reducing pain in neonates. For example, H. N. Yashwanth Raju et al [18] found that lingual 25% dextrose is a safe and effective analgesia in neonates undergoing minor invasive procedures. Similarly, expressed breast milk has also been shown to be effective in reducing pain in neonates. For example, Sr Lalitha Rosali et al [21] found that expressed breast milk significantly reduces pain during ROP screening.
However, the current study’s results suggest that 25% dextrose may be a more effective pain relief option for neonates undergoing blood sampling. The scoring system used was NIPS, as it is reliable, objective and approved for use in term neonates. A detailed analysis was conducted to compare the effectiveness of two pain relief interventions, 25% D and EBM in neonatal pain management during blood sampling, as assessed by the Neonatal Infant Pain Scale (NIPS). The statistical comparison using an independent T-test revealed a significant difference between the two groups. The mean NIPS score for the DEX group was notably lower compared to the EBM group(T-statistic -2.52) and p-value of 0.014 Table-2. This p-value, being well below the threshold of 0.05, indicates that the observed difference is unlikely to have occurred by chance.Such results hold clinical significance These findings align with prior research by Rawal et al. [8] and Danilyn M. Angeles et al. [25], which highlight the efficacy of sweet solutions such as oral dextrose in pain relief by activating endogenous opioid pathways.
Conclusion
This study was a hospital‑based Pre experimental Study. The participants of this study were neonates between ≥34 weeks and ≤42 weeks who met the inclusion criteria and had an indication of blood sampling by Venipuncture. They were randomized into two groups, with Group-1 of 30 neonates receiving EBM and Group-II of 30 neonates receiving 25% Dextrose. The procedure selected was venipuncture, which is routinely done as indicated. For neonates who received expressed breast milk, the mean pain score was 3.40 ±1.26. In contrast, neonates administered 25% dextrose had a lower mean pain score of 2.67 ± 0.96. This indicates that the average pain experienced by neonates in the 25% D group was lower than that in the EBM group. These findings suggest that Oral 25% dextrose is significantly more effective than expressed breast milk in reducing neonatal pain during venepuncture.
Recommendations
(a). The intervention of administration of 25% dextrose can be incorporated into standard operating protocols of NICU.
(b). Systematic Review can be conducted.
Conflict of Interest: None
Source of funding: None
Authors’ Contributions: BP: Problem statement, Aims, Objectives, Methodology, Publication; BP, RKF: Data Collection, Review of literature, Discussion and References; BP, RKF, KC: Statistical Analysis, Results Writing, Conclusions; and SAS: Data Analysis, Results Writing, and interpretation. BP, RKF, KC and SAS: All authors have written, red and accepted to publish the manuscript.
Here, BP: Brinda P; RKF: Reetha KF; KC: Keka Chatterjee; SAS: Saritha AS
References
- Johnston C. Neonatal pain: A journey spanning three decades. Paediatric Neonatal Pain 2020;2:339. PMID: 8975195
- Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am 1989;36:795-822. PMID: 2565344
- Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: A systematic review. Clin J Pain 2015;31:355-62. PMID: 25507131
- Boggini T, Pozzoli S, Schiavolin P, Erario R, Mosca F, Brambilla P, et al. Cumulative procedural pain and brain development in very preterm infants: A systematic review of clinical and preclinical studies. Neurosci Biobehav Rev 2021;123:320-36. PMID: 33108312
- Taddio A, Shah V, Hancock R, Smith RW, Stephens D, Atenafu E, et al. Effectiveness of sucrose analgesia in newborns undergoing painful medical procedures. CMAJ 2008;179:374-373. PMID: 18740121
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: Development and initial validation. Clin J Pain 1996;12:13-22. PMID: 8701896
- Bilgen H, Ozek E, Cebeci D, Ors R. Comparison of sucrose, expressed breast milk, and breastfeeding on the neonatal response to heel prick. J Pain 2001;2:301-305. PMID: 14615676
- Rawal S, Ghai A, Jindal T. Twenty-five per cent dextrose and EBM in pain relief during heel lance in late preterm babies using the PIPP score: A randomized controlled trial. J Neonatol 2018;32:439. PMID: 57916965
- Sahoo JP, Rao S, Nesargi S, Ranjit T, Ashok C, Bhat S. Expressed breast milk versus 25% dextrose in procedural pain in neonates, a double-blind randomized controlled trial. Indian Pediatr 2013;50:203-207. PMID: 23551424
- Stevens B, Yamada J, Campbell-Yeo M, Gibbins S, Harrison D, Dionne K, et al. The minimally effective dose of sucrose for procedural pain relief in neonates: A randomized controlled trial. BMC Pediatr 2018;18:85. PMID: 29684555
- Neshat H, Jebreili M, Seyyedrasouli A, Ghojazade M, Hosseini MB, Hamishehkar H. Effects of breast milk and vanilla odors on premature neonate’s heart rate and blood oxygen saturation during and after venipuncture. Pediatr Neonatol 2016;57:225-231. PMID: 26952734
- Upadhyay A, Aggarwal R, Narayan S, Joshi M, Paul VK, Deorari AK. Analgesic effect of expressed breast milk in procedural pain in term neonates: A randomized, placebo-controlled, double-blind trial. Acta Paediatr 2004;93:518-522. PMID: 15160096
- Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, et al. The premature infant pain profile-revised (PIPPR): Initial validation and feasibility. Clin J Pain 2014;30:238-243. PMID: 24332738
- Jonsdottir RB, Kristjansdottir G. The sensitivity of the premature infant pain profile – PIPP to measure pain in hospitalized neonates. J Eval Clin Pract 2005;11:598-605. PMID: 16207373
- Nayak R, Nagaraj KN, Gururaj G. Prevention of pain during screening for retinopathy of prematurity: A randomized control trial comparing breast milk, 10% dextrose and sterile water. Indian J Pediatr 2020;87:353-358. PMID: 32132322
- Abinaya S, Ramesh S. Does oral 25% dextrose effectively reduce procedural pain in neonates–A randomized controlled trial. JMSCR 2017;5:3062731. DOI: https://dx.doi.org/10.18535/jmscr/v5i11.140
- Varghese TC, Paul AS, Soans S. Oral dextrose versus breast milk for pain relief in newborns infants. Clin Invest 2020;10:904.
- Yashwanth RH, Sudha R, Shwetha BN. Effect of oral 25% dextrose on pain relief in newborn infants undergoing venipuncture. Int J Contemp Pediatr 2020;7(4):891-895. PMID: 2020114
- Shanthi, et al. Efficacy of 10% dextrose vs. expressed breast milk in relieving procedural pain. Journal of Clinical Neonatology 2024;13(3). DOI: https://doi.org/4103/jcn.jcn_37_24
- Ou-Yang MC, Tsao PN, Chen CY, Hsieh WS, & Chen CH. Randomized controlled trial of expressed breast milk for relieving pain in preterm infants during heel-lancing. Pediatrics 2012;129(4):657-664. PMID: 22431832
- Rosali SL, Nesargi S, Mathew S, & Rao SP. Efficacy of expressed breast milk in reducing pain during retinopathy of prematurity screening: A randomized controlled trial. Indian Pediatrics 2014;51(8):635-638. PMID: 25120592
- Gupta SK, Saha S, & Kumar P. Randomized controlled trial on the efficacy of oral dextrose for pain relief in neonates during blood sampling. Journal of Neonatal-Perinatal Medicine 2011;4(2):145-150.
- Gharehbaghi MM, & Ali P. The effect of oral dextrose on pain relief of newborn infants: A randomized controlled trial. Pakistani Journal of Biological Sciences 2007;10(9):1465-1469. DOI: https://doi.org/1203/00006450-201011001-00477
- Carbajal R, Veerapen S, Couderc S, Jugie M, & Ville Y. Analgesic effect of breastfeeding in term neonates: Randomized controlled trial. BMJ 2003;326(7379):13-15. PMID: 12538452
- Angeles DM, Ashwal S, Sternberg M, McGorray S, Weiner S, Zimmerman, AW, & Maron, B. S. (2020). Oral dextrose reduces procedural pain without altering cellular ATP metabolism in preterm neonates: A prospective randomized trial. Journal of Perinatology 2020;40(1):88-95. PMID: 31699677
- Lago P, Garetti E, Bellieni CV, Merazzi D, Ancora G, Pirelli A & Pain Study Group of the Italian Society of Neonatology. Systematic review of pain management practices for neonates in NICUs across Europe. Pediatrics 2009;123(6):eee566-eeee573. PMID: 19490464
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, et al. Cortical pain responses in human infants. The Journal of Neuroscience 2010;30(14):4917-4921. PMID: 20386531
- Kasaab MI, El-Sayed MI, & Abd El-Aziz MS. The effectiveness of glucose in reducing needle-related procedural pain in neonates: A randomized controlled trial. Journal of Pediatric Nursing 2012;27(4):427-434. PMID: 22884243
- Malngiang B, Sing S, Golmei Net al. A comparative study between expressed breast milk and oral glucose for the relief of pain in newborns undergoing skin pricking procedures. IOSR J Dent Med Sci 2016;15(3):28-32. PMID: 22392169 DOI: https://doi.org/10.9790/0853-15312832
- Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychological Med 2013;35(2):121-126. PMID: 24049221
![]() This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
