![]()
Drug-induced Photosensitivity: Photoallergic, Phototoxic and Adverse Drug Reactions
Year: 2024; Volume: 4; Issue: 4; Page No: 23 – 28
Article Type: Short Communication
Authors: Bayan Omar Sharif 1*![]() , Naza Mohammed Ali Mahmood2
, Naza Mohammed Ali Mahmood2![]() , Sakar Bikhtiyar Saber3
, Sakar Bikhtiyar Saber3![]() , Danyar Akbar Muhamad4
, Danyar Akbar Muhamad4![]() , Shanar Jamal Rahim5
, Shanar Jamal Rahim5![]() , Frishta Ahmed Salih6
, Frishta Ahmed Salih6![]()
![]() https://doi.org/10.55349/ijmsnr.2024442328
https://doi.org/10.55349/ijmsnr.2024442328
Affiliations:
1Specialist Nurse, Sulaimani Directorate of Health, Health Development and Training Center, Kurdistan Region, Iraq.
2Assisstant Professor, University of Sulaimaniyah, College of Pharmacy, Pharmacology & Toxicology Department, Kurdistan Region, Iraq.
3Pharmacist, Shorsh General Hospital, Kurdistan Region, Iraq.
4 Nurse, Baxshin Hospital, Kurdistan Region, Iraq.
5Biologist, College of Science – Biology department, University of Sulaimani, Kurdistan Region, Iraq.
6 Pharmacist, Zad medical center, Iraq.
Corresponding Author:
Dr. Bayan Omar Sharif,
Sulaimani Directorate of Health,
Health Development and Training Center,
Kurdistan Region,
Iraq.
Email ID: omerbayn82@gmail.com
| How to cite this article: Sharif BO, Mahmood NMA, Saber SB, Muhamad DA, Rahim SJ, Salih FA. Drug-induced Photosensitivity: Photoallergic, Phototoxic and Adverse Drug Reactions. Int J Med Sci and Nurs Res 2024;4(4):23–28. DOI: 10.55349/ijmsnr.2024442328 |
Article Summary: Submitted: 25-October-2024 Revised: 28-November-2024 Accepted: 25-December-2024 Published: 30-December-2024
Abstract
Background: Drug-induced photosensitivity refers to skin reactions caused by exposure to ultraviolet light following the use of certain medications, which can be administered topically or orally. When ultraviolet radiation interacts with a chemical present in adequate concentrations within the skin, it may trigger various reactions, particularly in susceptible individuals. The most common responses are photoallergic and phototoxic reactions.
Methods: In this paper, we discussed about drug-induced photosensitivity: Photoallergic, phototoxic and adverse drug reactions, and some of those medication that leads to these conditions.
Results: Photosensitive drug eruptions are cutaneous adverse events due to exposure to a medication and either ultraviolet or visible radiation.
Conclusion: Photosensitive drugs (PSDs) represent an important research area and more investigations would be helpful to better predict drug photosensitizing potential, prevent and manage cutaneous adverse events and find the most appropriate alternative therapeutic strategy. As well as, various medications, particularly antibiotics and analgesics, can induce both photoallergic and phototoxic reactions, there are significant differences between the two, including their onset timing, requirement for prior exposure, underlying mechanisms, clinical presentation, and histopathological features.
Keywords: drug-induced photosensitivity, photoallergic, phototoxic, adverse drug reactions, medications
Full Text
Introduction
Drug-induced photosensitivity refers to a cutaneous condition that either develops or worsens due to ultraviolet (UV) exposure in combination with a chemical agent. Such exposure may occur through systemic or topical medications capable of penetrating the skin. Drug-induced photosensitivity is a relatively common phenomenon in clinical practice, accounting for approximately 8% of reported drug-related adverse skin reactions. [1] Photoallergic reactions are immunologically driven, requiring prior sensitization, and typically present similarly to allergic contact dermatitis. In contrast, phototoxic reactions do not necessitate prior sensitization and manifest as an exaggerated sunburn-like response. [2]
Both oral and topical medications can trigger photosensitive skin reactions when exposed to ultraviolet (UV) light. In predisposed individuals, photoallergy and phototoxicity arise when UV radiation interacts with chemicals concentrated in the skin. Although the current literature primarily comprises case reports and series, suggesting these occurrences are largely anecdotal, drug-induced photosensitivity remains a significant subset of drug-related cutaneous reactions, contributing to up to 8% of medication-associated adverse effects. [3] Although photoallergic and phototoxic reactions share some clinical features, they are distinguishable based on several key aspects.
Characteristics of photoallergic reactions include: (i) they are less prevalent than phototoxic responses; (ii) their occurrence is independent of medication dose or radiant energy; (iii) minimal exposure to the photosensitizing drug is sufficient; (iv) they develop at least 24 hours following initial exposure; (v) prior sensitization is essential; (vi) they present as eczematous dermatitis, often extending beyond UV-exposed areas; (vii) histopathological findings demonstrate epidermal spongiosis rather than necrosis; (viii) they are mediated by immune mechanisms; and (ix) they may be triggered by cross-reacting drugs with structural similarities. [2]
Material and Methods
Effects of ultra violet A and B (UVA and UVB)
The primary distinction between UVA and UVB rays lies in their wavelength and energy:
- UVA rays have longer wavelengths with lower energy, enabling them to penetrate deeper into the skin.
- UVB rays have shorter wavelengths with higher energy, but they penetrate the skin more superficially. [4]
Both UVA and UVB rays contribute to skin damage and skin cancer, but each causes distinct types of harm, necessitating protection from both. Sunscreen application is one of the most effective methods for shielding the skin against UV radiation. It is crucial to choose a sunscreen offering protection against both UVA and UVB rays. Historically, sunscreens primarily protected against UVB radiation due to the earlier understanding of its effects, but it is now widely recognized that UVA rays also pose a significant risk. Ultra violet B protection is quantified by the SPF (Sun Protection Factor) rating
indicated on sunscreen labels. Although there is no standardized system to measure UVA protection, many modern sunscreens incorporate ingredients to counter UVA radiation as well. Such sunscreens are commonly labeled as “broad-spectrum” or “UVA/UVB protection”, signifying their efficacy against both types of UV rays. However, as there is currently no universal standard defining the degree of UVA protection, these terms may lack precise meaning. [5]
Figure-1 Effectiveness of sunscreen ingredients depends on their ability to protect against ultraviolet radiation [6]
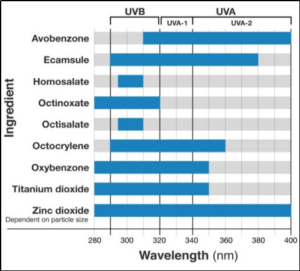
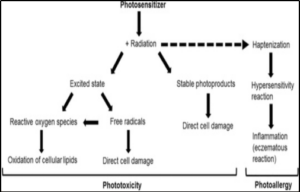
Figure-2 Schematic illustration of the major mechanisms of phototoxic and photoallergic tissue damage [3]
Adverse drug reactions (ADRs)
Adverse drug reactions (ADRs) represent a major public health concern, contributing to increased mortality, morbidity, and healthcare expenditures, including higher hospital admission rates and extended hospital stays. [7] Healthcare professionals (HCPs)—including physicians, pharmacists, dentists, and nurses—play a pivotal role in pharmacovigilance programs. However, under-reporting of ADRs remains widespread, especially in low- and middle-income countries (LMICs). [8]
Photosensitivity skin reactions with some class of medications
Photosensitivity skin reactions are often triggered by drugs (photosensitizers) administered either systemically or topically. Drug classes with high photosensitizing potential include antibiotics, diuretics, and nonsteroidal anti-inflammatory drugs (NSAIDs). Photosensitizers act as chromophores, typically possessing a planar, tricyclic, or polycyclic structure and a low molecular weight of 200–500 Da. [2]
These molecules are usually activated by ultraviolet (UV) light—most commonly UVA light (320–400 nm), which penetrates deeply into the dermis—or, less frequently, visible light. During this process, photosensitizers absorb radiant energy in the form of photons and enter an excited state, triggering photochemical reactions with other molecules in the epidermal and/or dermal tissues. These molecular interactions form the basis of photosensitivity reactions in the skin.
Based on the underlying pathophysiologic mechanisms, photosensitivity drug reactions are classified as either phototoxic or photoallergic. Phototoxic reactions are more common, can occur in any individual, and depend solely on the dose of the photosensitizer and the level of exposure to activating light wavelengths. In contrast, photoallergic reactions are rare, require prior sensitization, and occur independently of the photosensitizer dose. Many oral medications have been implicated in causing phototoxic skin eruptions, and some of these can also induce photoallergic responses. Notably, tetracyclines and quinolones are frequent causes of drug-induced phototoxicity. [2]
Incidence of Drug- Induced Phototoxic and Photoallergic Reactions
Data on drug-induced photosensitivity are primarily derived from national reporting systems, such as the US Food and Drug Administration (FDA) and the UK’s Committee on Safety of Medicines, with less frequent input from international sources like the WHO Program for International Drug Monitoring. Since reporting is typically voluntary, the data are often biased, incomplete, and disproportionately focused on newly marketed drugs. Despite these limitations, drug-induced photosensitivity is recognized as a relatively common event, accounting for up to 8% of all adverse drug reactions. [9]
Phototoxic reactions can affect patients of any age, though they are more commonly observed in women. These reactions typically resemble severe sunburn and are confined to areas exposed to sunlight. The hallmark symptoms include erythema and edema, which appear within minutes to a few hours after UV exposure. Vesiculation and blistering are rare. Persistent hyperpigmentation may occur, and skin damage can persist for years, even after the removal of the triggering drug.[12] In contrast, photoallergic dermatitis occurs more frequently in men and usually develops 24 to 72 hours post-exposure. It commonly affects sun-exposed areas such as the face, neck, upper chest, and hands, though lesions may spread to non-exposed regions. This condition presents with
symptoms similar to contact dermatitis, including desquamation and prolonged hyperpigmentation, which can persist for over a year. The predominant feature of photoallergic dermatitis is eczematous dermatitis. [12]
Table-1 Systemic medications causing phototoxicity and their action spectrum, selected [10]
| Class of drugs | Name of the drugs | Action of the spectrum |
| Antimicrobials | Doxycycline | UVA |
| Minocycline | UVA | |
| Ciprofloxacin | UVA | |
| Levofloxacin | UVA | |
| Voriconazole | Unknown | |
| Diuretics | Furosemide | Unknown |
| hydrochlorothiazide | UVA | |
| NSAIDs | Ketoprofen | UVA |
| Naproxen | UVA | |
| Others | Quinidine | UVA |
| Psoralens | UVA | |
| Retinoids | UVA, UVB | |
| Calcium channel blockers | UVA |
Table 2. Topical medications causing photoallergy and their action spectrum, selected [10]
| Class of drugs | Name of the drugs | Action of the spectrum |
| NSAIDs | Ketoprofen | UVA |
| Benzophenone | UVA | |
| Meloxicam | UVA | |
| Piroxicam | UV | |
| Others | Acyclovir | UVA |
| Hydrocortisone | UVA |
Table-3 Systemic medications causing photoallergy and their action spectrum, selected [10]
| Class of drugs | Name of the drugs | Action of the spectrum |
| Sulphur- containing drugs | Hydrochlorothiazide | UVA |
| Sulfadiazine | UVB | |
| Sulfonamides | UVB | |
| Sulfonylureas | UVA | |
| Antimicrobials | Chloramphenicol | Unknown |
| Lomefloxacin | UVA, UVB | |
| NSAIDs | Ketoprofen | UVA |
| Naproxen | UVA | |
| Phenotiazines | Chlorpromazine | UVA |
| Others | Dapsone | Unknown |
| Amantadine | UVA | |
| Ranitidine | UVA |
Table-4 Oral medications capable of causing phototoxicity [11]
| hototoxic | Name of the drugs | Action of the spectrum |
| Antimicrobials | Doxycycline | UVA |
| Lymecycline | UVA | |
| Minocycline | UVA | |
| Tetracycline | UVA | |
| Demeclocycline | UVA | |
| Ciprofloxacin | UVA | |
| Enoxacin | UVA | |
| Fleroxacin | UVA | |
| Levofloxacin | UVA | |
| Lomefloxacin | UVA and UVB | |
| Nalidixic acid | UVA | |
| Pefloxacin | UVA | |
| Sparfloxacin | UVA | |
| Griseofulvin | UVA | |
| Sulfur-containing medications | Hydrochlorothiazide | UVA |
| Sulfonamides | UVB | |
| UVA | UVA | |
| NSAIDs | Ketoprofen | UVA |
| Naproxen | UVA | |
| Suprofen | UVA and UVB | |
| Tiaprofenic acid | UVA | |
| Benzophenone | UVA | |
| Carprofen | UVA | |
| Others | Atorvastatin | UVB |
| Calcium-channel blockers | UVA | |
| Chlorpromazine | UVA | |
| Calcium channel blockers | UVA |
Diagnosis of photoallergy and phototoxicity
Drug-induced photoallergy and phototoxicity are diagnosed by correlating a history of photosensitizing drug use with the presence of sun-exposed skin eruptions. Differentiation between these conditions typically requires photo patch testing, minimal erythema dose (MED) testing, and, in some cases, skin biopsies. Photo patch testing involves applying suspected photoallergens to the skin in duplicate: one area is exposed to UVA light, while the other remains shielded. A plate glass barrier is used to block UVB contamination. Skin reactions are evaluated at 24, 48, and 72–96 hours post-exposure; a reaction confined to the UVA-exposed area confirms photoallergy. For suspected phototoxicity, skin areas not directly exposed to sunlight are irradiated with gradually increasing doses of both UVA and UVB to determine the Minimal Erythema Dose (MED). The MED represents the lowest dose of radiation required to induce visible skin redness. A response of erythema at a lower-than-expected MED indicates drug-induced phototoxicity. The MED typically returns to normal levels upon re-testing after two weeks. Skin biopsy can also be useful for differentiating between photoallergy and phototoxicity. Histopathological findings show that photoallergic reactions resemble allergic contact dermatitis, while phototoxic reactions mimic sunburn-like changes. [13]
Management of photoallergy and phototoxicity
Patients should be advised to limit excessive exposure to sunlight when prescribed medications with photosensitizing potential. Using broad-spectrum sunscreens that provide protection against both UVA and UVB rays can help reduce the risk of photosensitive reactions. If photosensitivity occurs, management typically involves symptomatic relief, such as the use of cool compresses, soothing lotions, topical corticosteroids, and systemic antipruritic agents. The most definitive approach is to discontinue the causative medication; however, it is important to note that photosensitivity may persist for several months or even years after stopping the drug. [2]
Conclusion
Photosensitive drugs (PSDs) are a crucial area of research, and further studies are needed to improve the prediction of their photosensitizing potential, prevent and manage skin-related adverse effects, and identify suitable alternative treatment options. Additionally, while various medications—especially antibiotics and analgesics-can cause both photoallergic and phototoxic reactions, these two conditions differ significantly in terms of onset time, the need for prior exposure, underlying mechanisms, clinical manifestations, and histopathological characteristics.
Conflict of Interest – None
Source of funding – None
Authors’ Contributions
BOS – Study design and data collection, NMAM – Study design, concepts, data collection, BOS, NMAM – Data collection and analysis, BOS, SBS – Data collection, Manuscript writing, DAM – Manusript writing, and BOS, NMAM, SBS, DAM, SJR and FAS – All authors were wrote the manuscript, reading and checking all the aspects and approved by all authors.
BOS – Bayan Omar Sharif; NMAM – Naza Mohammed Ali Mahmood; SBS – Sakar Bikhtivar Saber; DAM – Danyar Akbar Muhamad; SJR – Shanar Jamal Rahim; FAS – Frishta Ahmed Salih
References
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clinics in dermatology 2016;34(5):571-581. DOI: https://doi.org/10.1016/j.clindermatol.2016.05.006
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert opinion on drug safety 2007; 6(4):431-443. DOI: https://doi.org/10.1517/14740338.6.4.431
- Hofmann GA, Weber B. Drug‐induced photosensitivity: culprit drugs, potential mechanisms and clinical consequences. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2021;19(1):19-29. DOI: https://doi.1111/ddg.14314
- Lee WJ, Chae SY, Ryu HS, Jang YH, Lee SJ. Inflammatory cytokine expression and sebum production after exposure of cultured human sebocytes to ultraviolet a radiation and light at wavelengths of 650 nm and 830 nm. Annals of dermatology 2015;27(2):163-170. DOI: https://doi.org/10.5021/ad.2015.27.2.163
- Rai R, Srinivas CR. Photoprotection. Indian Journal of Dermatology, Venereology and Leprology 2007; (73):73. DOI: https://doi.org/10.4103/0378-6323.31889
- Dan Kern, 2024 Available at: DOI: https://www.acne.org/whats-the-difference-between-uva-and-uvb-rays
- Mouton JP, Njuguna C, Kramer N, Stewart A, Mehta U, Blockman M, Fortuin-De Smidt M, De Waal R, Parrish AG, Wilson DP, Igumbor EU. Adverse drug reactions causing admission to medical wards: a cross-sectional survey at 4 hospitals in South Africa. Medicine 2016; 95(19): e3437. DOI: https://doi.org/1097/MD.0000000000003437
- Oumar AA, Dakouo M, Tchibozo A, Maiga M, Landouré G, Abdi-Bogoreh R, Tulkens PM, Dao S, Yombi JC. Antiretroviral-induced adverse drug reactions in HIV-infected patients in Mali: a resource-limited setting experience. International journal of basic and clinical pharmacology 2019;8(5):831. DOI: https://doi.org/10.18203/2319-2003.ijbcp20191565
- Nakao S, Hatahira H, Sasaoka S, Hasegawa S, Motooka Y, Ueda N, Abe J, Fukuda A, Naganuma M, Kanoh H, Seishima M. Evaluation of drug-induced photosensitivity using the Japanese Adverse Drug Event Report (JADER) database. Biological and Pharmaceutical Bulletin 2017; 40(12):2158-2165. DOI: https://doi.org/10.1248/bpb.b17-00561
- Glatz M, Hofbauer GF. Phototoxic and photoallergic cutaneous drug reactions. InAdverse Cutaneous Drug Eruptions 2012; (97):167-179). Karger Publishers. DOI: https://doi.org/10.1159/000335630
- Vandecasteele SJ, Van Wijngaerden E, Peetermans WE. Two cases of severe phototoxic reactions related to long-term outpatient treatment with voriconazole. European Journal of Clinical Microbiology and Infectious Diseases 2004; (23): 656 -657. DOI: https://doi.org/10.1007/s10096-004-1176-7.
- Lugović-Mihić L, Duvančić T, Ferček I, Vuković P, Japundžić I, Ćesić D. Fotoosjetljivost uzrokovana lijekovima–kontinuirani dijagnostički izazov. Acta clinica Croatica 2017;56(2):277-283. DOI: https://doi.org/10.20471/acc.2017.56.02.11
- Millard TP, Hawk JL. Photosensitivity disorders: cause, effect and management. American journal of clinical dermatology 2002;(3):39-46. DOI: https://doi.org//10.2165/00128071-200203040-00002
![]() This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
