![]()
Comparison of the two administration modalities of subcutaneous enoxaparin injection on formation of ecchymosis
Year: 2025; Volume: 5; Issue: 1; Page No: 20 – 23
Article Type: Original Article
Rakhi Sudarsanan1*![]() , Reena Kumari2
, Reena Kumari2![]() , Keka Chatterjee3
, Keka Chatterjee3![]() , Ayappan S Saritha4
, Ayappan S Saritha4![]()
![]() https://doi.org/10.55349/ijmsnr.2025512023
https://doi.org/10.55349/ijmsnr.2025512023
Affiliations:
1Critical Care Nurse, College of Nursing, INHS Asvini, Colaba, Mumbai, India. Email ID: rakhivs82512@gmail.com
2Clinical Tutor, College of Nursing, INHS Asvini, Colaba, Mumbai, India. Email ID: gudia.reena@gmail.com
3Vice Principal, College of Nursing, INHS Asvini, Colaba, Mumbai, India. Email ID: chatterjeeez@gmail.com
4Principal, College of Nursing, INHS Asvini, Colaba, Mumbai, India. Email ID: sarithas330@gmail.com
| How to cite this article: Sudarsanan R, Kumari R, Chatterjee K, Saritha AS. Comparison of the two administration modalities of subcutaneous enoxaparin injection on formation of ecchymosis. Int J Med Sci and Nurs Res 2025;5(1):20–23. DOI: 10.55349/ijmsnr.2025512023 |
Article Summary: Submitted: 15-January-2025 Revised: 30-January-2025 Accepted: 27-February-2025 Published: 31-March-2025
Abstract
Background: Enoxaparin, a low-molecular-weight heparin, is commonly used in critical care to prevent thromboembolic disorders. However, this drug has certain side effects such as subcutaneous bruising, which is known to be the most common adverse effect of this drug. Objectives of the study were to assess the effect of administration protocol of subcutaneous enoxaparin injection on formation of ecchymosis and to find the association between administration protocol of subcutaneous enoxaparin injection on formation of ecchymosis.
Materials and Methods: A Cross over design was used to compare the two administration modalities of subcutaneous enoxaparin injection on formation of ecchymosis. Ethical clearance and formal permission to conduct the study was obtained. A self-designed observation checklist was used after validation. Initial injection was administered for durations 10 sec with immediate withdrawal of needle (Control intervention) and the next injection was administered for durations of 30 sec with a 10 sec delayed needle withdrawal (Experimental intervention) to the same patient after 12 hrs. The size of ecchymosis was measured in cm and was compared after 24 hrs & 48 hrs post administration. The collected data was analysed by using descriptive and inferential statistics.
Results: The comparison of ecchymosis size after 48 hours between two subcutaneous enoxaparin injection administration modalities revealed that control intervention resulted in significantly larger ecchymosis sizes compared to experimental intervention.
Conclusion: From this study, we have concluded that the 30-second injection technique with delayed withdrawal is recommended to minimize ecchymosis formation and improve patient comfort.
Key Words: Enoxaparin, subcutaneous injection, ecchymosis, anticoagulation, nursing care
Full Text
Introduction
Low-molecular-weight heparin (LMWH), derived from unfractionated heparin (UFH), is widely used for preventing thromboembolic conditions, especially in acutely ill hospital patients. [1] Nurses play a critical role in administering these drugs correctly, monitoring side effects, and ensuring compliance. [2] Reducing patients’ discomfort and concerns whenever and wherever possible is an important aim of nursing. [8] One common side effect of subcutaneous LMWH, like enoxaparin, is ecchymosis (bruising), with incidences reported as high as 88.9%. Although bruising is benign, it can cause discomfort, limit injection sites, and lead to anxiety and non-compliance. [3] The healthcare group should manage medication intervention to achieve the primary goal of drug therapy, i.e. maximizing the beneficial effects with minimal harm to the patient. The importance of this role of nurses is more prominent when drugs are administered by following the proper administration protocols. [4] For example, enoxaparin sodium is injected subcutaneously in many patients admitted to hospitals, including those with ischemic heart disease, with the aim of preventing the progression of complications and its administration continues until discharge from hospital. [5] The factors affecting the local bruising due to subcutaneous Enoxaparin injection include Injection site, administration modality, injection time, and rubbing time after injection. Among them, improper method of removing needle from skin is an important reason for subcutaneous bruising. Proper administration techniques are essential to minimize ecchymosis, enhance patient comfort, and improve care standards in clinical settings. [3]
Materials and Methods
A Crossover Randomized Controlled Trial (RCT) was used to compare the two administration modalities of subcutaneous enoxaparin injection on formation of ecchymosis among 100 patients selected by convenient sampling (following the inclusion & exclusion criteria) out of 250 patients receiving injection Enoxaparin as a part of their treatment protocol in a critical care unit of tertiary care hospital during the data collection period. A pilot study was conducted to evaluate the feasibility, methods, and potential challenges of a research were excluded from the main study. The initial Enoxaparin injection was administered for 10 sec durations with immediate withdrawal of needle (Control Intervention) and the next injection was administered for 30 sec durations with a 10 sec delayed needle withdrawal (Experimental Intervention) to the same patient after 12 hrs. as shown in Table–1.
Table – 1 Distribution of Control, Study groups and its specifications
| Groups | Control group | Study group |
| Intervention | Subcutaneous Injection of Enoxaparin | Subcutaneous Injection of Enoxaparin |
| Time period | Within 10 sec | For 30 sec |
| Withdrawal Time | Immediate withdrawal of needle | 10 Sec delayed withdrawal of needle |
| Observation of Ecchymosis | 48 hours post Injection | 48 hours post Injection |
Administration Protocol
Injection Enoxaparin was administered at a 90° angle to the lateral abdominal area, about 5 cm around the navel, with the duration timed using a wristwatch. [3] The initial Enoxaparin injection was administered for 10 sec durations with immediate withdrawal of needle and the subsequent injections were alternated between sides of the abdomen every 12 hours with 30 sec durations with a 10 sec delayed needle withdrawal.
The site was marked with a waterproof marker to avoid overlap and bruise sizes were measured after 48 hours using transparent paper and graph paper.
Ethical Considerations and Statement
Ethical clearance and formal permission to conduct the study was obtained from the Hospital Administration vide School of Naval Medicine, SRC No. 86/2024. Written informed consent was taken from the study participants ensuring voluntary participation. Purpose of the study was explained to the study participants and confidentiality was assured.
Sample size calculation
The sample size was calculated using G*Power software based on a two-tailed test.
Sampling Technique: Randomized allocation of patients to different intervention sequences.
Sample Size Calculation: Based on power analysis to ensure statistical significance.
Data Management and Statistical Analysis
The data were organized, tabulated, analysed, and interpreted using descriptive and inferential statistics. The findings were organized and presented in two parts with tables and figures. Results were computed by using Microsoft Excel 2010 and IBM SPSS 27.0 version, based on the objectives of the study.
The demographic data was analysed in frequency and percentage. Each subscale under section was analysed out of corresponding frequency, mean and standard deviation using descriptive statistics. The association of demographic variables with observation checklist was done using inferential statistics Chi-Square test.
Results
The comparison of ecchymosis size after 48 hours between two subcutaneous enoxaparin injection administration modalities revealed that the 10-second immediate withdrawal protocol resulted in significantly larger ecchymosis sizes compared to the 30-second with 10-second delay withdrawal protocol.
Figure-1 Multiple bar chart depicting the percentage distribution of study participants by the Ecchymosis Size after 48 Hours of Enoxaparin injection between 10-Second administration modality and 30-Second with 10-Second delayed withdrawal modality (N=100)
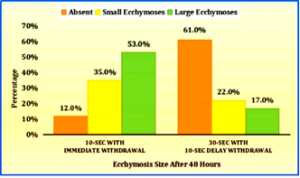
The above graph illustrates the comparison of ecchymosis size after 48 hours between two subcutaneous enoxaparin injection administration modalities. Among the control arm, majority (53.0%) experienced large ecchymosis (more than 2 cm). Additionally, 35.0% had small ecchymosis (1 to 2 cm), and 12.0% had minimal ecchymosis (below 1 cm). Whereas, in experimental arm majority (61.0%) had no/ minimal (below 1 cm) ecchymosis, followed by 22.0% with small ecchymosis (1 to 2 cm) and 17.0% with large ecchymosis (more than 2 cm) as shown in Figure–1.
Figure-2 Pie Chart showing the Percentage Distribution of the Sample by Dose of Injection Enoxaparin (N=100)
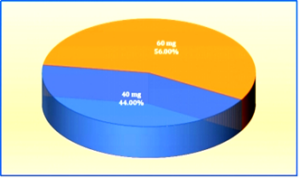
The graph shows that 56% of participants received a 60mg dose, while 44% received a 40mg dose. In the control arm, the mean ecchymosis size for the 60 mg dose was 2.65 cm, compared to 2.17 cm for the 40 mg dose and statistically significant with p = 0.035 (p<0.05). Similarly, in the experimental arm, the mean ecchymosis size for the 60 mg dose was 1.40 cm, compared to 0.89 cm for the 40 mg dose and statistically significant with p = 0.002 (p<0.05).
Table–2 Comparison of mean betweenn Ecchymosis Size by Injection Techniques
|
Injection administration protocol |
n |
Mean± SD | df |
p-value |
| 10-sec with
immediate withdrawal |
100 |
2.44 ± 1.22 |
198 |
<0.001* |
| 30-sec with
10-sec delay withdrawal |
100 |
1.17 ± 0.82 |
The difference in ecchymosis formation between the two techniques was calculated by independent samples t-test which indicates statistically highly significant with p<0.001, indicating a reduction in ecchymosis size with the 30-second technique.
Discussion
Anticoagulation has proved to be an important treatment modality in preventing thrombus formation. Local side effects of heparin injection include pain, erythema and ecchymosis. [9] The prevalence of complications depends on the injection site, technique, and drug absorption. [10] In this study the finding suggested that administration of Injection Enoxaparin in 30 sec duration with 10 sec delay in withdrawal of needle was effective in reducing ecchymosis (2.44±1.22 and 1.17±0.82 Mean and SD respectively). The study findings are supported by the Randomized controlled trial done by Sommapun Jueakaew et al among 44 patients with acute deep vein thrombosis. [6] Bruises occurred in 50.0% and 18.2% of control and study group patients, respectively (p = 0.03). Mean bruise size between 48 and 60 h after injection was 172.73 ± 372.60 mm2 and 28.18 ± 70.01 mm2 in the control group and study group, respectively and significant with p = 0.026.
Senay Uzun Aciksoz et al also studied the Effect of Administration Protocol of Subcutaneous Enoxaparin Injection on Formation of Ecchymosis. [7] Mean bruise size was 125.3 and 22.0 in the control group and study group, respectively. Here both studies found that slower injection and delayed needle withdrawal (e.g., 30-second injection and 10-second withdrawal delay) led to a significant reduction in ecchymosis size compared to faster injection methods.
The rationale in both studies is that slower administration reduces tissue trauma and allows for better diffusion of the drug. This suggests that clinicians need to adopt a more tailored approach when administering subcutaneous anticoagulants, taking both technique and patient-specific characteristics into account.
Conclusion
The study contributes valuable insights into the role of injection technique, dose of enoxaparin, and patient history in the formation of ecchymosis following subcutaneous enoxaparin administration. The findings suggest that modifying the administration modality to a 30-second injection with a 10-second delay can effectively reduce the size of ecchymosis, particularly in patients receiving higher doses of the drug. These results underscore the importance of individualized patient care and the careful consideration of clinical and procedural factors to minimize adverse outcomes in patients undergoing anticoagulant. Adopting this technique in critical care units may help in improving patient comfort and minimize injection site ecchymosis.
Limitations of the study
- Limited Sample Size: The study had a relatively small sample size, which can limit the statistical power of the findings. A larger sample would enhance the robustness of the results and allow for more nuanced analysis of subgroups.
- Variability in Injection Sites: Differences in injection sites (e.g., abdomen vs. thigh) or variations in the administration technique (e.g., angle of injection) not have been accounted for, potentially affecting the outcomes.
- Duration of the study period was short.
Conflict of Interest: None
Source of Funding: None
Acknowledgement
The authors gratefully acknowledged the patients who generously agreed to participate in this study. Heartfelt gratitude to Mrs. Jilmy Anu Jose, Mrs. Linu Zachariah and Mrs. Ampili who have validated my tool and for their valuable input. Mr. Ananthakrishnan and Mr Bharat Sambhyal, the Cardiac physicians for granting permission to conduct the study in the cardiac critical care unit. Mrs. Swati Dalvi, Mrs. Krishnendhu and Miss. Nisha the nursing officers working in cardiac unit for their valuable suggestions and guidance. Mr. Vivek Hande the Director and Mrs. Fancy Xavier the Nursing Superintendent of the hospital for their constant guidance and encouragement
Authors’ Contributions
RS: study design, data collection and data analysis; RS and RG: Manuscript writing and analysis. KC and ASS: Revising critically for important intellectual content. All authors approved the final version to be submitted.
Here, RS: Rakhi Sudarsanan; RK: Reena Kumari; KC: Keka Chatterjee; and ASS: Ayappan S Saritha
References
- Solari F, Varacallo MA. Low-Molecular-Weight Heparin (LMWH). Treasure Island (FL): StatPearls Publishing; 2025. PMID: 30247832
- Hanson A, Haddad LM. Nursing rights of medication administration. Treasure Island (FL): StatPearls Publishing; 2025. PMID: 32809489
- Dehghani K, Najari Z, Dehghani H. Effect of subcutaneous enoxaparin injection duration on bruising size in acute coronary syndrome patients. Iranian Journal of Nursing and Midwifery Research 2014; 19(6):5645-68. PMID: 25558251
- Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. PMID: 21328752
- Jupalli A, Iqbal AM. Enoxaparin [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. PMID: 30969687
- Jueakaew S, Piancharoensin R, et al. Novel subcutaneous low-molecular-weight heparin injection technique to reduce post-injection bruising: A randomized controlled trial. Phlebology 2018;33(9):618-624. PMID: 30453827
- Aciksoz SU, Sözbilir A, Korkmaz A, Yildirim Y, Balcı D. Effect of administration protocol of subcutaneous enoxaparin injection on formation of ecchymosis: An experimental study. J Clin Nurs 2016;25(19-20):3004-3011. PMID: 27028689
- Mohammady M, Janani L, Akbari Sari A. Slow versus fast subcutaneous heparin injections for prevention of bruising and site pain intensity. J Clin Nurs 2017;26(21-22):3384-3389. DOI:1111/jocn.13710. PMID: 28965359
- Simeon I, Thenmozhi P. Dry cold application on pain and ecchymosis among patients receiving low molecular weight heparin. Res J Pharm Technol 2021;14(11):5825-5829. DOI: 52711/0974-360X.2021.01007.
- Bayram SB, Gulnar E, Aksoy F. The effect of two types of subcutaneous heparin injections on pain, ecchymosis, hematoma and drug absorption: a quasi-experimental study. J Eval Clin Pract 2025;31(1):e14266. DOI: 1111/jep.14266. PMID: 39660578
![]() This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑Non-Commercial‑ShareAlike 4.0 International License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given, and the new creations are licensed under the identical terms.
